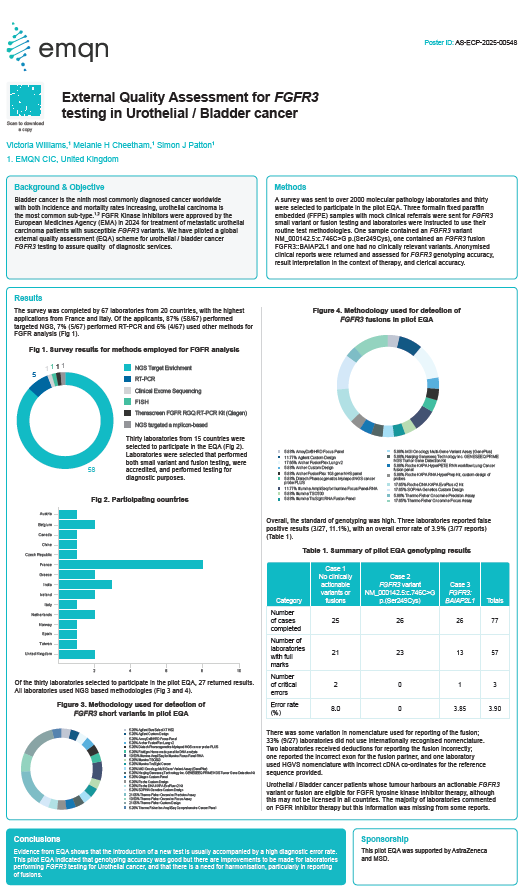

External Quality Assessment for FGFR3 testing in Urothelial / Bladder cancer
Authors: Victoria Williams, Melanie H Cheetham, Simon J Patton
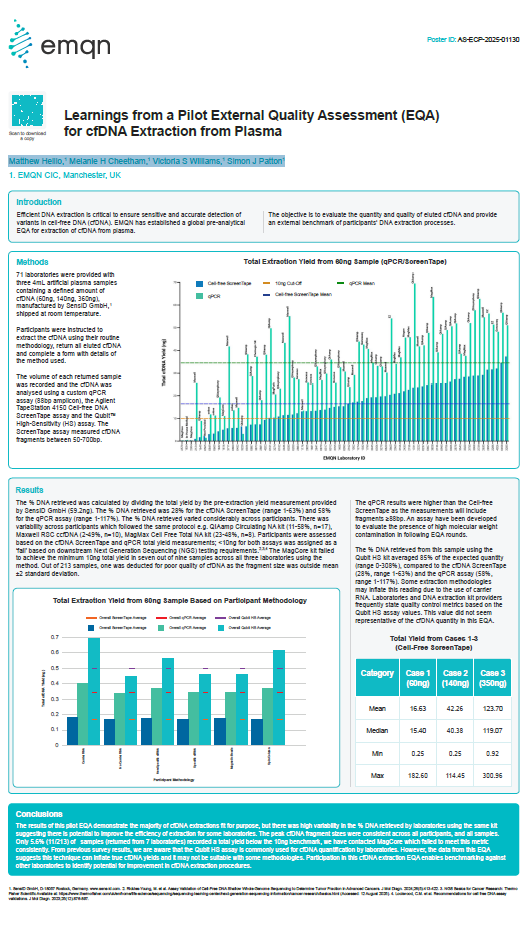
Learnings from a Pilot External Quality Assessment (EQA) for cfDNA Extraction from Plasma
Authors: Matthew Hellio, Melanie H Cheetham, Victoria S Williams, Simon J Patton
External Quality Assessment (EQA) for Homologous Recombination Deficiency (HRD) testing in ovarian cancer: Findings of a new international Quality Network for Pathology (IQN Path) pilot scheme
Authors: Arfa Maqsood, Victoria Williams, Francesca Fenizia, Jennifer A Fairley, Tracy Stockley, Sze Chai, Etienne Rouleau,Simon J Patton




We hope to see you there!